For doctors
TREATswitzerland at a glance
| Objective: | Investigation of medical care and response to treatment |
| Enrolment: | Patients aged 12 and over with moderate and severe atopic dermatitis |
| Data: | Demographics, previous therapy and course of the therapy, severity of atopic dermatitis, current therapy, efficacy, side effects, patient-related outcomes (PROs) |
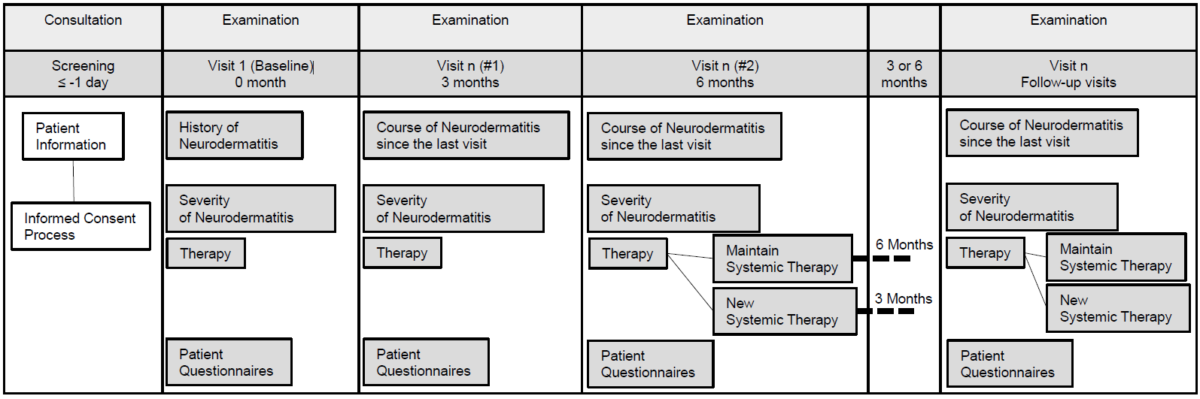
| Procedure: | At the start, after 3 and 6 months, subsequently at three or six-month intervals, during routine examinations |
| Observation: | At least 24 months |
| Cohort: | 700 patients |
1. Objectives
Since there is no structured and detailed data on the treatment of atopic dermatitis (AD) for Switzerland to date, a registry is intended to remedy this situation. It will provide data on the medical care of patients with AD for health care research and enable studies on the effectiveness and safety of approved AD therapies in everyday life.
The primary objective of the TREATswitzerland registry project is to create a disease-focussed, prospective cohort of patients with moderate and severe AD.
The primary objective is to document the medical care of patients with moderate and severe AD in order to be able to evaluate the treatment in terms of its effectiveness and suitability.
Additional objectives of the AD registry are as follows:
- To assess the psychosocial impact of AD
- To provide up-to-date epidemiological data, which enables investigation of risk factors for favourable or unfavourable disease courses and comorbidities; and
- To establish a research network and promote clinical research projects.
2. Implementation
The TREATswitzerland registry study is sponsored by the SSDV. It is set up and managed by the main study site, the Department of Dermatology at the Inselspital, Bern University Hospital.
Study sites are to be set up at university hospitals, cantonal hospitals and practices throughout Switzerland, in which patients with AD will be cared for and enrolled in the registry.
The study sites participating in TREATswitzerland are as follows:
- Department of Dermatology, Inselspital, Bern University Hospital
Prof. Dr. med. Dagmar Simon
- Dermatology & Skin Care Clinic, Ennetbürgerstrasse 36, 6374 Buochs
PD Dr. med. Ahmad Jalili
- Universitätsspital Basel, Petersgraben 4, 4031 Basel
Prof. Dr. med. Karin Hartmann, Leiterin Allergologie
- PLAZA Kliniken, Oberlandstrasse 100, 8610 Uster
Dr. med. Tobias Plaza
- Lucerne Cantonal Hospital, Center for Dermatology and Allergology, 6000 Lucerne 16
Prof. Dr. med. Christoph Brand
- Kantonsspital St. Gallen, Klinik für Dermatologie, Venerologie und Allergologie
Rorschacherstrasse 95, 9007 St. Gallen
Ieva Saulite, MD
- Kantonsspital Freiburg / HFR Hôpital Cantonal de Fribourg, Dermatology
Chem. des Pensionnats 2/6, 1752 Villars-sur-Glâne
Dr. med. Basile Page
3. Study design
TREATswitzerland is a prospective, national, multicenter registry for adolescents and adults (patients aged 12 and over) suffering from moderate to severe AD.
Inclusion criteria:
- Age ≥ 12 years
- AD according to the diagnostic criteria of the UK Working Party
- Moderate to severe AD defined as
- Objective SCORAD > 20 or IGA ≥ 3 or
- Currently undergoing systemic anti-inflammatory therapy for AD or
- Previous systemic anti-inflammatory therapy for AD within the last 24 months
4. Sample size
The aim is to enrol 700 patients and to observe each of them for at least 2 years.
5. Visits
6. Study criteria
Questionnaires on demographics and AD:
- Medical history (general, AD specific)
- Examination and documentation of the skin status (severity: SCORAD, EASI, global assessment of the investigator, body surface)
- Documentation of the prescribed therapy, reasons for the treatment decision, adverse drug effects of the AD therapy
Patient questionnaires:
- Sociodemographic characteristics
- History of AD, therapy adherence
- Quality of life (DLQI, CDLQI)
- AD symptoms (POEM, pruritus NRS, sleep disturbance NRS)
- AD disease course (number of weeks with complete/good disease control in the last 12 weeks)
- Disease-related impairment of professional/educational ability
- Symptoms of depression (CES-D)
- Fatigue (FSS)
- enefits and requirements of therapy (PBI; patient benefit index), satisfaction with treatment.
7. Organisation
The start of the study is planned for Q2 2022.
Following a pilot phase at the Department of Dermatology, the other study sites are gradually being included.
8. Procedure
Wherever possible, the registry visits should be carried out together with the routine check-ups. This is a non-interventional study. The treatment of the patients is determined according to their condition and the severity of the AD and is the responsibility of the treating dermatologists at the study site.
If severe side effects occur as a result of the therapy, the treating doctors at the study site must report these to Swissmedic via BASEC within 7 days (HRO Art. 21).
The following steps are necessary prior to enrolment of each patient:
- Reviewing the inclusion criteria
- Obtaining patient consent
The data for the TREATswitzerland registry is collected electronically. The REDcap® data collection tool used for this purpose is operated by SwissRDL at the Institute of Social and Preventive Medicine at the University of Bern.
In the study site, the data from the visit/medical examination can be entered and saved online in the TREATswitzerland registry database.
During the visit, the patients are given a QR code allowing them to access the questionnaires, which they can complete directly on a tablet.
9. Management
The TREATswitzerland AD registry is managed by the Swiss Society of Dermatology and Venereology (SSDV) (sponsor). The cooperation between TREATswitzerland and the study sites is governed by agreements
The principal investigator is Prof. Dr med. Dagmar Simon, Inselspital, Bern University Hospital.
The project is monitored by a scientific advisory committee made up of representatives from the study sites. This committee coordinates and decides on the subsequent use of the registry data for research purposes and its publication.
10. Publication
The registry data should be evaluated and published as follows:
- Annual report on demographic data and treatments to the SSDV
- Annual report on demographic data and treatments to the sponsors as well as company-specific information on the nature and number of treatments with their medications. The transfer of data is contractually agreed between the SSDV and the industrial partners.
- Scientific publications.

Principal Investigator
Prof. Dr. med. Dagmar Simon